First immunotherapy approved for type 1 diabetes
In Clinical
Follow this topic
Bookmark
Record learning outcomes
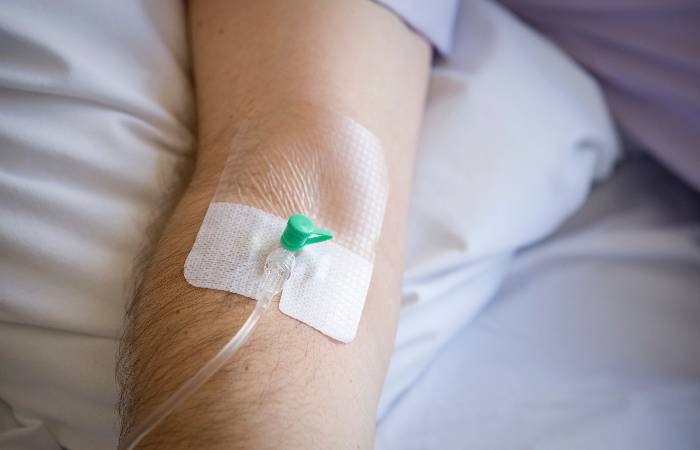
The MHRA has approved teplizumab (Tzield), the UK’s first immunotherapy for type 1 diabetes, marking what has been called a “turning point” in treatment.
The therapy is licensed for adults and children aged eight years and over with stage 2 type 1 diabetes, a point at which people are at high risk of developing stage 3 – when blood sugar problems typically emerge, requiring lifelong insulin.
Administered via a 14-day course of daily intravenous infusions, teplizumab works by inhibiting the immune system’s attack on insulin-producing beta cells. Clinical evidence suggests it can delay progression to stage 3 diabetes by an average of three years.
The approval, made under the International Recognition Procedure, comes as the prevalence of type 1 diabetes continues to rise. Diabetes UK welcomed the decision, with director of research Dr Elizabeth Robertson describing it as a “breakthrough moment” that could offer “precious extra years free from the relentless demands” of the condition.
However, teplizumab is not yet available on the NHS. NICE’s draft guidance, published earlier this month, said more evidence is needed before recommending routine use. A final decision is expected in November.